Study of the cyclization of N-hydroxy- and N-methoxy-N-(2-oxoalkyl)amidesIvan V. Kulakov, Irina V. Palamarchuk, Elena B. Nikolaenkova, Alexsei Ya.Tikhonov, Yuriy V. Gatilov, and Alexander S. Fisyuk Institute of Chemistry, Tyumen State University, Tyumen, Russia
E-mail: i.v.kulakov@utmn.ru Received: 10 December 2020 Accepted: 22 April 2021 Abstract: AbstractEfficient methods for the preparation of 1-hydroxy-1,5-dihydro-2H-pyrrol-2-ones and 1-hydroxy-1,6-dihydropyridine-2,5-diones on the basis of N-hydroxy-N-(2-oxoalkyl)amides are reported. The reaction of N-hydroxy-N-(2-oxoalkyl)amides in the presence of 3 eq. of t-BuOK was found to lead to 1-hydroxy-1,5-dihydro-2H-pyrrol-2-ones. A decrease in the base content to 1.5 eq. or less leads to increase in 1-hydroxy-3-phenyl-1,6-dihydropyridine-2,5-dione content in the reaction products due to oxidation of the starting compounds by atmospheric oxygen and their subsequent transformation. Using the examples of chloroacetohydroxamic acids, we demonstrated the possibility of nucleophilic substitution and subsequent cyclization to 1-hydroxy-1,5-dihydro-2H-pyrrol-2-one derivatives. To study the possible mechanism of radical cyclization of N-hydroxy-N-(2-oxoalkyl) amides to 1,6-dihydropyridine-2,5-dione, the supposed reactive center (hydroxamic group) was blocked. It was shown that the protection of the N-hydroxy group by methylation of N-hydroxy-N-(2-methyl-3-oxobutan-2-yl)-2-phenylacetamide to N-methoxy-2-phenylacetamide in its further reaction with 0.5 eq. of t-BuOK results in the formation of intermediate 4-hydroxy-1-methoxy-3-phenylpyrrolidin-2-one. The desired 1-methoxy-1,5-dihydro-2H-pyrrol-2-one was obtained when using an excess of t-BuOK. Formation of N-methoxy-1,6-dihydropyridine-2,5-diones as a result of radical oxidative cyclization described earlier was not detected. Graphic Abstract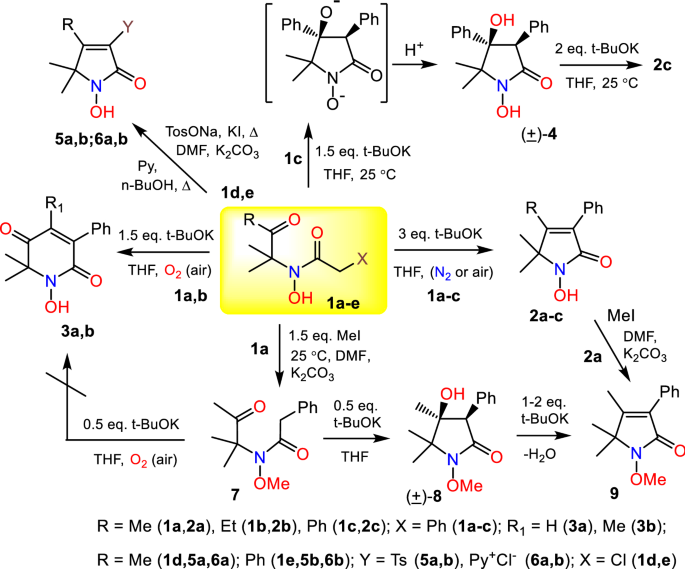 Keywords: Hydroxamic acid; Intramolecular cyclization; Oxidation reaction; 1,5-dihydro-2H-pyrrol-2-ones; 1,6-dihydropyridine-2,5-diones; X-ray structural analysis Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-021-01673-0
Chemical Papers 75 (9) 4517–4525 (2021) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||





