2H-Pyrano[3,2-c]quinolin-2-ones: their convenient synthesis and selected reactionsMatúš Čakurda, Pavol Koiš, Gabriela Addová, Margita Lácová, and Andrej Boháč Comenius University in Bratislava, Bratislava, Slovakia
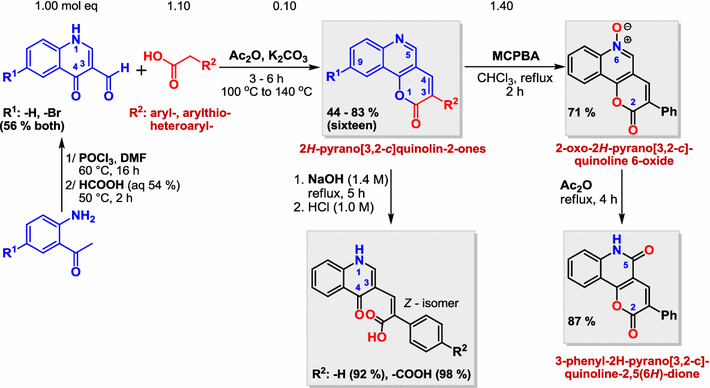
E-mail: andrej.bohac@fns.uniba.sk Abstract: Despite the structure attractiveness of 2H-pyrano[3,2-c]quinolin-2-ones 3 their synthesis is not sufficiently developed. Only 35 pyranoquinolinones 3 are registered in the SciFinder database. Unavailability of 3 limits their chemistry exploitation, physical and biological studies. We have developed a convenient and general methodology for the synthesis of 3. Sixteen novel 2H-pyrano[3,2-c]quinolin-2-ones 3 were prepared by a cyclocondensation of easily available 4-oxo-1,4-dihydroquinoline-3-carbaldehydes 1 with monosubstituted acetic acids 2 (-aryl, -arylthio and -heteroaryl). To support chemistry exploitation of pyranoquinolinones 3, oxoquinolinylphenylacrylic acids 4 were obtained by hydrolysis of 3 with NaOH (92–98%). A simple oxidation of 3 by MCPBA was performed to provide oxopyranoquinoline N-oxide 5 (71%). Convenient rearrangement of 5 in refluxing Ac2O curried out 2H-pyrano[3,2-c]quinoline-2,5(6H)-dione 6 in 87% yield. Moreover, some of the prepared pyranoquinolinones 3 possess intensive blue fluorescence properties. Here we described the simple and general synthesis that allows availability of 2H-pyrano[3,2-c]quinolin-2-ones 3. Some transformations of 3 to the novel heterocyclic compounds 4–6 were performed as well in good yields (71–98%). The synthesis of 6 from 3 was not yet described. The developed methodology for the synthesis of 3–6 can stimulate their further physical and pharmacological studies.
Keywords: 2H-Pyrano[3,2-c]quinolin-2-one ; (Z)-3-(4-Oxo-1,4-dihydroquinolin-3-yl)-2-phenylacrylic acid ; 2-Oxo-2H-pyrano[ Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-017-0319-0
Chemical Papers 72 (3) 683–690 (2018) |
Saturday, April 26, 2025 |
|||
© 2025 Chemical Papers |
||||