Efficient synthesis of aurone Mannich bases and evaluation of their antineoplastic activity in PC-3 prostate cancer cellsAntonina V. Popova, Mykhaylo S. Frasinyuk, Svitlana P. Bondarenko, Wen Zhang, Yanqi Xie, Zachary M. Martin, Xianfeng Cai, Michael V. Fiandalo, James L. Mohler, Chunming Liu, David S. Watt, and Vitaliy M. Sviripa National Academy of Science of Ukraine, Kyiv, Ukraine
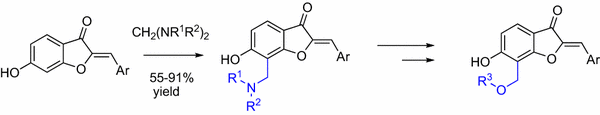
E-mail: vitaliy.sviripa@uky.edu Abstract: An efficient method for regioselective synthesis of C-7 Mannich bases of 6-hydroxyaurones was accomplished by the N,N-dialkylaminomethylation using aminals prepared from dimethylamine, dipropylamine, bis(2-methoxyethyl)amine, N-methylbutylamine, N-methylbenzylamine, morpholine, piperidine, and 1-methylpiperazine. Further transformation of 7-(N,N-dialkylamino)methyl group in these aurones led to formation of C-7 acetoxymethyl and methoxymethyl derivatives of 6-hydroxyaurones, some of which showed promising inhibition of PC-3 prostate cancer cell proliferation in the high nanomolar to low micromolar range that exceeded that of cisplatin. Compound 12c (R3 = Ac, Ar = 3,4-OMePh) displays 75% inhibition of PC-3 prostate cancer cells proliferation at 300 nM concentration. Keywords: 6-hydroxyaurones ; Mannich base ; Aminomethylation ; Prostate cancer Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0485-8
Chemical Papers 72 (10) 2443–2456 (2018) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||