Effect of synthesis method on structural properties and soot oxidation activity of gadolinium-doped ceriaAnjana P. Anantharaman, Hemanth J. Gadiyar, Mythili Surendran, A. Sumadhura Rao, Hari Prasad Dasari, Harshini Dasari, and G. Uday Bhaskar Babu National Institute of Technology Karnataka, Mangalore, India
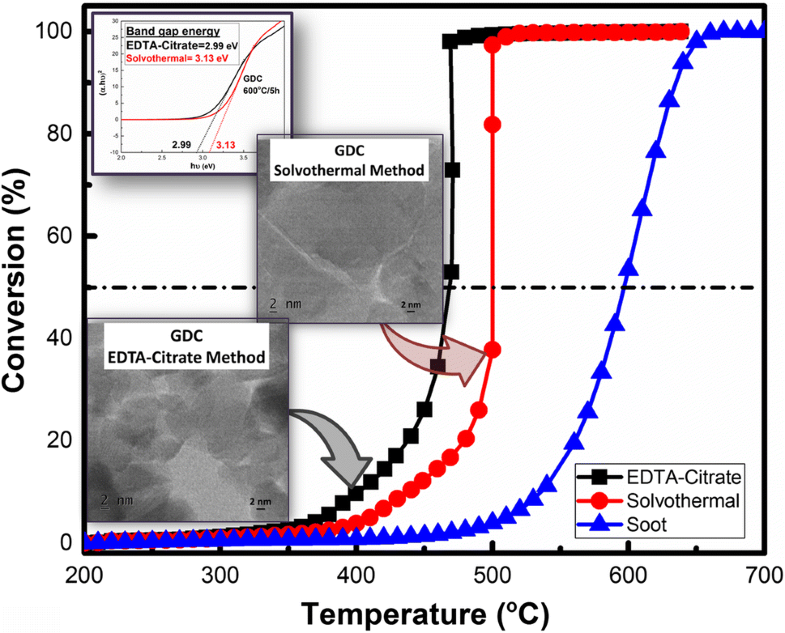
E-mail: energyhari@nitk.edu.in Abstract: EDTA–citrate complex and solvothermal methods were adopted for the synthesis of gadolinium-doped ceria (GDC) (Ce0.9Gd0.1O2) solid solution, and the obtained GDC sample is tested for soot oxidation activity. Based on XRD results, it was evident that the reactive facet planes of {100} and {110} were highly intense [intensity ratio (%) of (200)/(100) (34.2%) and (220)/(111) (51.2%)] for GDC prepared by the EDTA–citrate method in comparison with the solvothermal method, and this highly intense reactive facet plane correlates to the lower energy for oxygen vacancy formation. Apart from the smaller crystallite size (10 nm) the GDC sample prepared by the EDTA–citrate method displayed lower band gap energy (2.99 eV), higher ratio of reducibility (45.45%) and lower binding energy (528.8 eV) for surface-adsorbed oxygen. The GDC obtained by EDTA–citrate method displayed a better soot oxidation activity (T50 = 468 °C) than compared to the GDC obtained by solvothermal method (T50 = 500 °C). The obtained results significantly show that the synthesis method plays a crucial role in controlling the structural properties and in enhancing the soot oxidation activity. Keywords: Gadolinium-doped ceria (GDC) ; EDTA–citrate complexing method ; Solvothermal method ; Oxygen vacancy ; Soot oxidation activity Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0532-5
Chemical Papers 72 (12) 3179–3188 (2018) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||