A novel and efficient synthesis of 3-aryl-5-(2-hydroxybenzoyl)pyridin-2(1H)-ones by re-cyclization of N-(oxopyranochromenyl)acetamides and their antineoplastic screeningMargita Lácová, Matúš Čakurda, Pavol Koiš, Gabriela Addová, and Andrej Boháč Comenius University in Bratislava, Bratislava, Slovakia
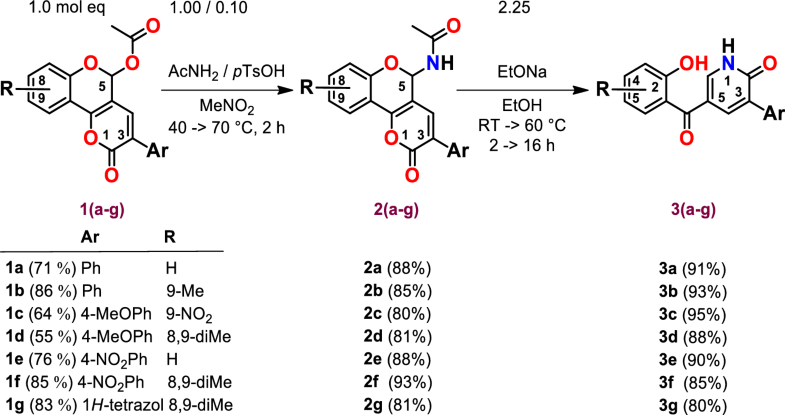
E-mail: andrej.bohac@fns.uniba.sk Abstract: We have developed a novel, simple and efficient methodology for preparation of yet unknown 3-aryl-5-(2-hydroxybenzoyl)pyridin-2(1H)-ones 3(a–g) by EtONa driven re-cyclization of N-(3-aryl-2-oxo-2,5-dihydropyrano[3,2-c]chromen-5-yl)acetamides 2(a–g) in good yields (80–95%). A mechanism for this reaction was proposed. The 3-aryl-2-oxo-2,5-dihydropyrano[3,2-c]chromen-5-yl acetates 1(a–g) were prepared by cyclocondensation from 3-formylchromones (4-oxo-4H-chromene-3-carbaldehydes) and acetic acids in 64–86% yields. Acetamides 2(a–g) were obtained by reaction of 3-aryl-2-oxo-2,5-dihydropyrano[3,2-c]chromen-5-yl acetates 1(a–g) with AcNH2 catalyzed by pTsOH in 80–93% yields. Click chemistry precursors 4(a,d) and 5(a,d) were prepared by propargylation of 3(a,d) in 92–98% yields. They can serve for construction of more complex molecules possessing pyridone skeleton of 3. Eleventh novel compounds 3(a–g), 4(a,d) and 5(a,d) were screened on their anticancer activity on a panel of human tumour cell lines by NCI USA. We found that pyridones 3–5 selectively inhibit the growth of some of the tumour cell lines at 10−5 M (up to -33% compared to a control). The most sensitive tumour cell lines originated from kidney, breast, skin, ovary, blood and lung. Keywords: 3-aryl-5-(2-hydroxybenzoyl)pyridin-2(1H)-ones ; N-(3-aryl-2-oxo-2,5-dihydropyrano[3,2-c]chromen-5-yl)acetamides ; re-cyclization mechanism ; NCI tu Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0566-8
Chemical Papers 73 (1) 55–69 (2019) |
Thursday, April 03, 2025 |
|||
© 2025 Chemical Papers |
||||