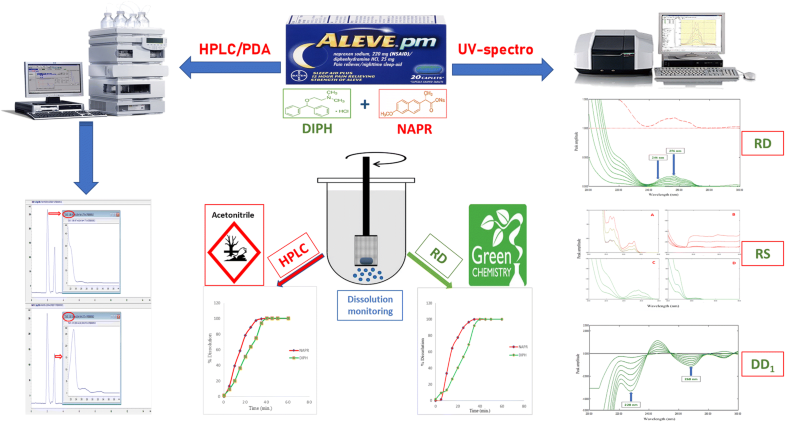
UV-spectrophotometry versus HPLC–PDA for dual-drug dissolution profiling: which technique provides a closer step towards green biowaiver concept? Novel application on the recent FDA-approved mixture Aleve pmAmal M. Abou Al-Alamein, Mohamed K. Abd El-Rahman, Esraa M. Fawaz, and Ezzat M. Abdel-Moety Cairo University, Cairo, Egypt
E-mail: esraa.fawaz@pharma.cu.edu.eg Abstract: Dissolution testing is a very significant tool that adds in vivo relevance to in vitro analytical data, thus providing a realistic in vitro/in vivo correlation. Dissolution profiling serves as a predictor of biological performance since the rate limiting step in the absorption of any drug is the rate of release from its pharmaceutical formulation. Being a routine procedure in quality control laboratories for initial approval process and scaling-up, the development of more and more eco-friendly methods for dissolution monitoring is considered as a worldwide trendy goal. In the present contribution, a comparison was highlighted between the two analytical techniques of utmost importance in acquisition of dissolution profiles: UV-spectrophotometry and HPLC–PDA focusing on the greenness of each one for further consolidation of the biowaiver concept. Both techniques were applied on the recently FDA-approved combination naproxen sodium (NAPR) and diphenhydramine hydrochloride (DIPH) formulated as Aleve pm® tablets. For the first time, this binary mixture was analyzed by three UV-spectrophotometric methods. NAPR was directly determined by zero-order spectrophotometry at 330 nm, where the spectrum of DIPH shows zero contribution. Whereas DIPH was determined by three simple methods exploiting the ratio spectra calculated using the spectrum of 20 µg/ml NAPR as a divisor. The first proposed method was ratio difference (RD), the second was ratio subtraction, and the last one was derivative ratio method. Being the simplest method, RD was the spectrophotometric method of choice that was applied in monitoring the dissolution of DIPH from Aleve pm® tablets. Although RD method has been widely described in many publications dealing with pharmaceutical analysis, it is the first article that opens a new horizon for RD method in the way of being a real-life application rather than only a method for determination. The second technique was a previously published HPLC–PDA, in which dissolution was then monitored by calculating the peak area of each component drug over time. Methods’ validation was performed agreeing with the ICH guidelines. The advantages and challenges of each technique are discussed in a side-by-side comparison giving a key to recognize which one can positively influence environmental well-being. Keywords: Naproxen ; Diphenhydramine ; UV-spectrophotometry ; Ratio difference ; HPLC–PDA ; Dissolution ; Biowaiver ; Green analytical chemistry Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0580-x
Chemical Papers 73 (2) 309–319 (2019) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||