Isomers of halodrol: synthesis and partial reduction to form A-ring of a long-term metabolite of dehydrochloromethyltestosterone (oral turinabol)Yurii Yu. Kozyrkov, Dmitri Yu. Shostko, and Sergey A. Beliaev National Anti-Doping Laboratory, Lesnoy, Belarus
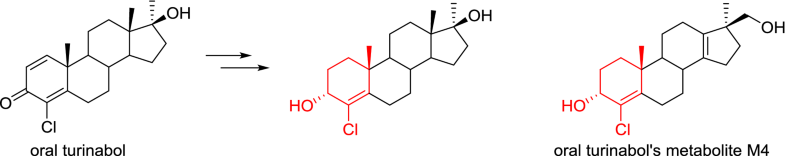
E-mail: kozyrkov@antidoping.by Abstract: A new synthetic route to individual isomers of 4-chloro-17α-methylandrost-1,4-diene-3,17β-diol (halodrol, CDMA) based on reduction of the carbonyl group in dehydrochloromethyltestosterone (oral turinabol, DHCMT), followed by separation of epimers by fractional crystallisation from dichloromethane is presented. Reduction of the disubstituted double bond of the diene system in 3α-hydroxy isomer of halodrol afforded 4-chloro-17α-methyl-androst-4-ene-3α,17β-diol contained A-ring corresponding to 4-chloro-17β-hydroxymethyl-17α-methyl-18-norandrosta-4,13-dien-3α-ol (M4), which is one of the long-term metabolites of oral turinabol. Keywords: Dehydrochloromethyltestosterone ; Oral turinabol ; Halodrol ; Partial reduction ; Isomers separation Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-018-0603-7
Chemical Papers 73 (3) 731–736 (2019) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||