Application of acid-promoted UiO-66-NH2 MOFs in the treatment of wastewater containing methylene blueYunxia Fang, Liuxue Zhang, Qianqian Zhao, Xiulian Wang, and Xu Jia Zhongyuan University of Technology, Zhengzhou, People’s Republic of China
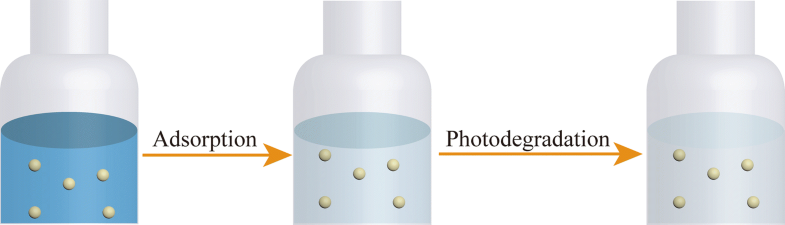
E-mail: zlx100100@163.com Abstract: A series of Zr-based MOFs were successfully prepared by conventional solvothermal method and microwave irradiation method. The effects of synthesis methods and acid modifiers on the morphology and thermal stability of the materials were investigated. The samples were characterized by powder X-ray diffraction, scanning electron microscopy, thermogravimetric analysis, N2 adsorption–desorption, Fourier transform infrared, and diffuse reflectance spectra. The results showed that the products synthesized by microwave irradiation possessed a better dispersibility and a lager specific surface area than those synthesized by solvothermal method. As a potential adsorbent for wastewater treatment, the adsorption and removal of cationic dye methylene blue (MB) by the synthetic materials was studied. The addition of acid as a regulator could increase the specific surface area of the materials, thereby improving the adsorption efficiency of MB. After repeated adsorption and desorption, the adsorption capacity of the samples could be regenerated by photocatalytic degradation. The photodegradation and regeneration experiments showed that the samples had good stability and reusability. All these indicated that this type of materials had great potential in the field of wastewater treatment and resource utilization. Keywords: Synthesis method ; Acid regulator ; Adsorption ; Resource utilization ; Regeneration and reusability Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-019-00692-2
Chemical Papers 73 (6) 1401–1411 (2019) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||