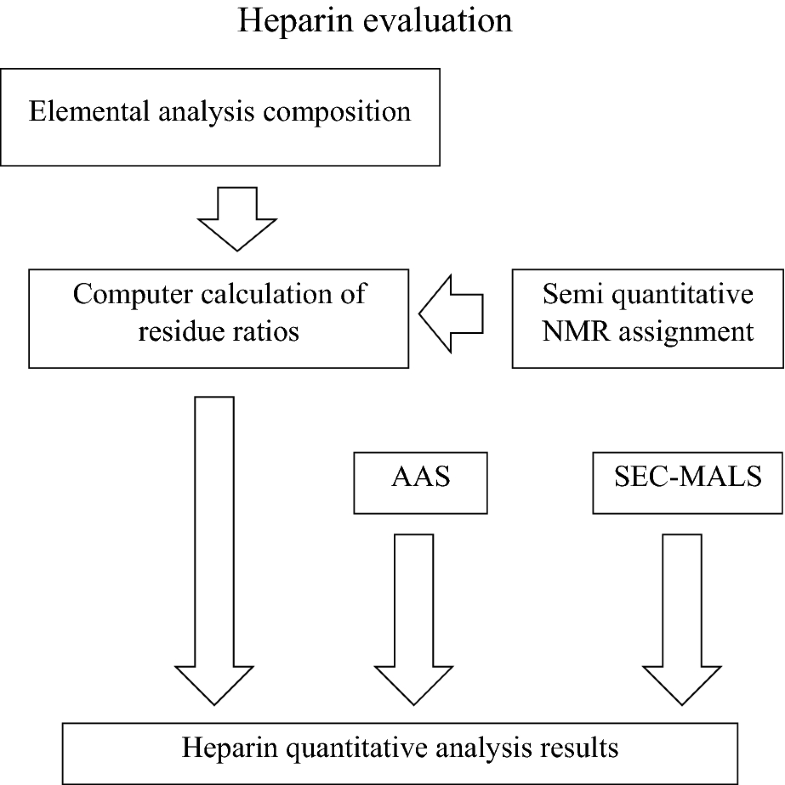
Heparin composition: calculation based on elemental analysis and NMR dataIvan Šimkovic, Michal Raab, Raniero Mendichi, Alena Manová, Alberto Giacometti Schieroni, and Miloš Hricovíni Slovak Academy of Sciences, Bratislava, Slovakia
E-mail: chemsimk@savba.sk Abstract: The analysis of many polysaccharides is often complicated due to the heterogeneity in their structures. A computer approach is presented exploiting the experimental data from elemental analysis and high-resolution NMR experiments to estimate the individual anhydro-unit ratio. Our approach is based on the fact that elemental composition of a particular type of saccharide is the same for saccharide units, connected by glycosidic bonds forming the polysaccharide chain, or for the individual saccharide anhydro structures complemented by the relevant amount of water molecules. From the possible structures generated, the structure is chosen by the computation with the best fit (“score”) to the elemental composition. The method was tested on a heparin pentasaccharide using the 1% deviation of HSQC-integrated limits at 5% water content. The optimal limits for evaluation of heparin in various ionic forms (Na+, Li+, or NH4+) for the computation input were found by the HSQC integration of the anomeric signals and anhydro unit ratios calculated based on elemental analysis, AAS, and SEC-MALS analysis. The results of heparin from different sources, and in various ionic environments, indicate that the presented methodology could be used as the complementary approach to other experimental analytical techniques of heparin and heparin sulfate. Keywords: Heparin ; Computer program ; Elemental analysis ; NMR ; AAS ; SEC-MALS Full paper is available at www.springerlink.com. DOI: 10.1007/s11696-019-00957-w
Chemical Papers 74 (1) 349–355 (2020) |
Monday, May 19, 2025 |
|||
© 2025 Chemical Papers |
||||